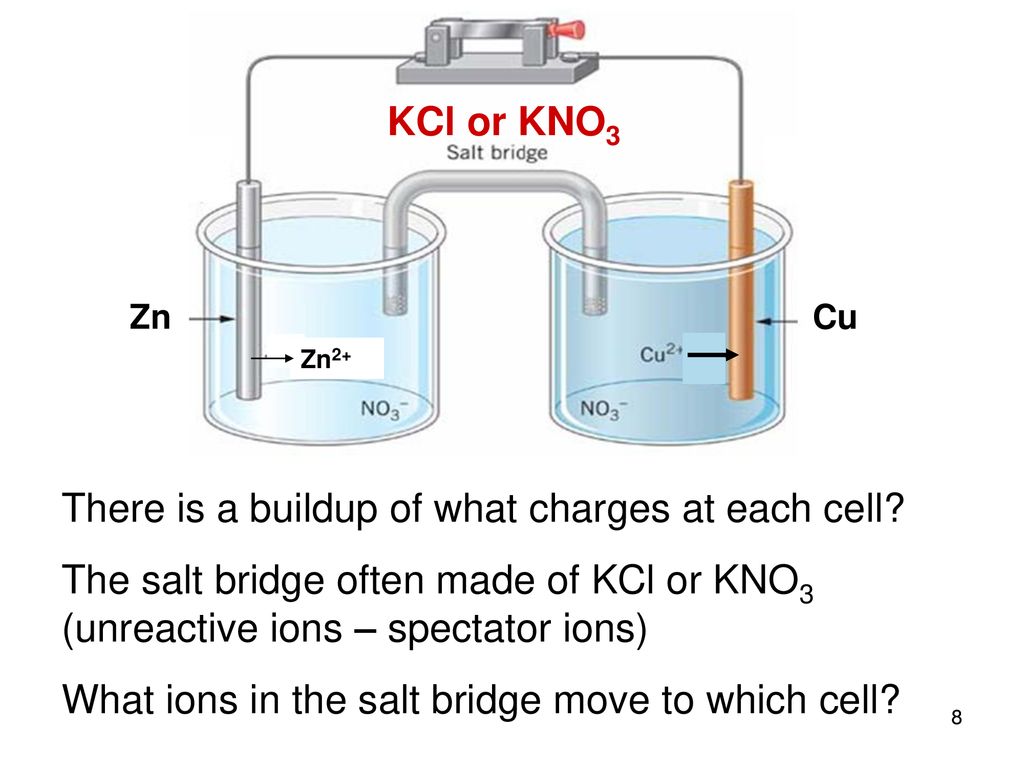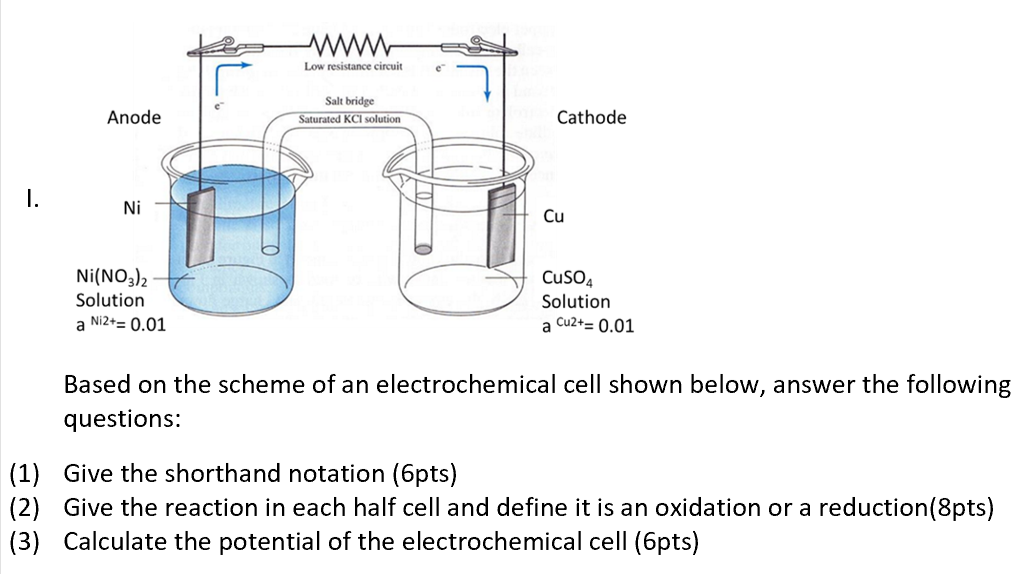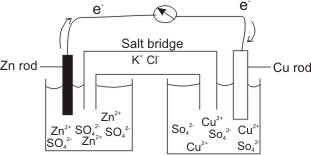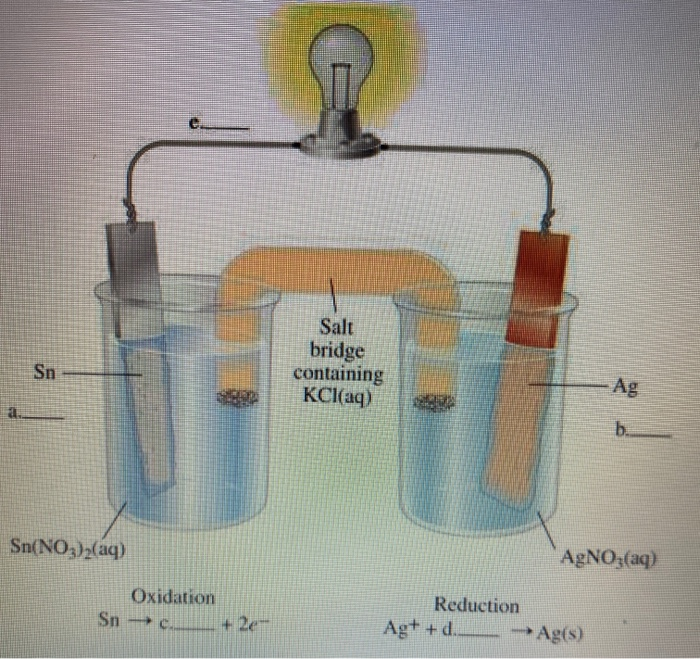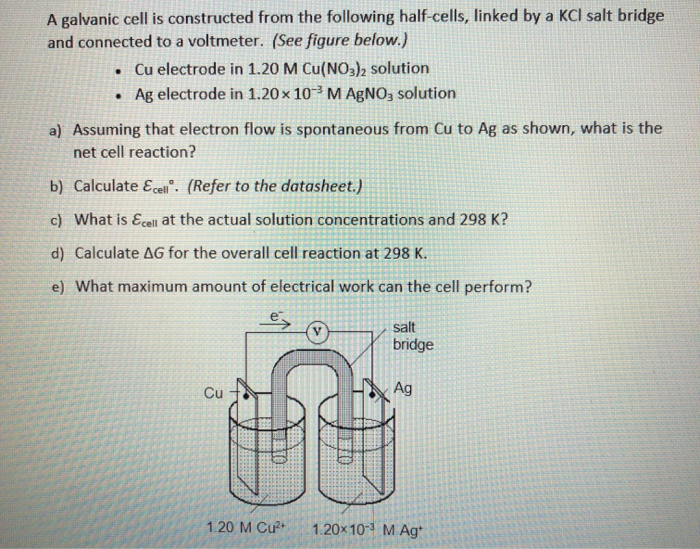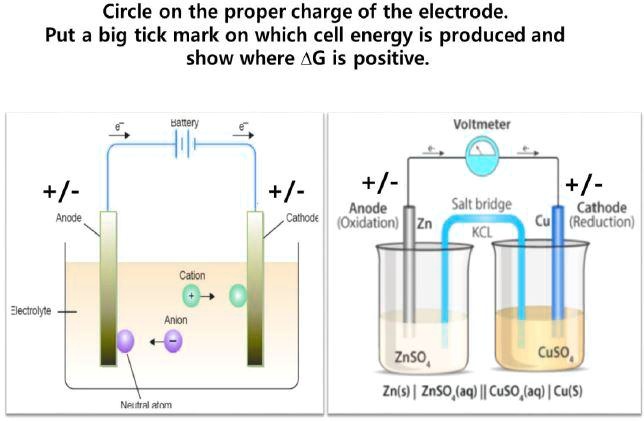
SOLVED: Circle on the proper charge of the electrode: Put a big tick mark on which cell energy is produced and show where AG is positive: Bacery Voltmeter +/- Anode +/- +/=

Schematic Diagram of a Potentiometric Electrochemical Cell | Image and Video Exchange ForumImage and Video Exchange Forum

KCl cannot be used as a salt bridge for the cell Cu(s) abs(CuSO(4)(aq))abs(AgNO(3)(aq)) Ag(s) because:

A salt bridge is used in voltaic cells to balance the ions and complete the circuit. Describe this scenario. (hint;use a diagram) | Homework.Study.com

Reference Electrodes with Salt Bridges Contained in Nanoporous Glass: An Underappreciated Source of Error | Analytical Chemistry
The function(s) of salt bridge in a cell is\/areA. It maintains standard electrode potential of cell constant which depends on several factors.B. It completes the electrical circuit.C. It departs both the solutions

physical chemistry - Why is it important to use a salt bridge in a voltaic cell? Can a wire be used? - Chemistry Stack Exchange

A) Schematic of the micro-agar salt bridge. Three percent agarose in 3... | Download Scientific Diagram