
SOLVED: The following information is given for magnesium at 1 atm: boling point=1090C Hyap1090C=5424J/g melting point=649.0C H649.0C=368.3J/g specific heat solid=1.017 g.C specific heat liquid=1.339 J g.c A 32.00 g Sample of liquid

Question Video: Understanding the Difference in Boiling and Freezing Points between Magnesium Chloride and Sodium Chloride Solutions of the Same Concentration | Nagwa

Magnesium. Alkaline earth metals. Chemical Element of Mendeleev's Periodic Table. in square cube creative concept Stock Photo - Alamy

The melting points of the Period 3 metals sodium and magnesium are shown below. What is the differences in the melting points of sodium and magnesium, using the model of metallic bonding?
Why Magnesium has least melting amd boiling point among group 2 elements? And why calcium has least density?

Magnesium Parodic Table Element Boiling Melting Stock Vector (Royalty Free) 1846322134 | Shutterstock

inorganic chemistry - Why is the melting point of magnesium oxide higher than aluminium oxide? - Chemistry Stack Exchange
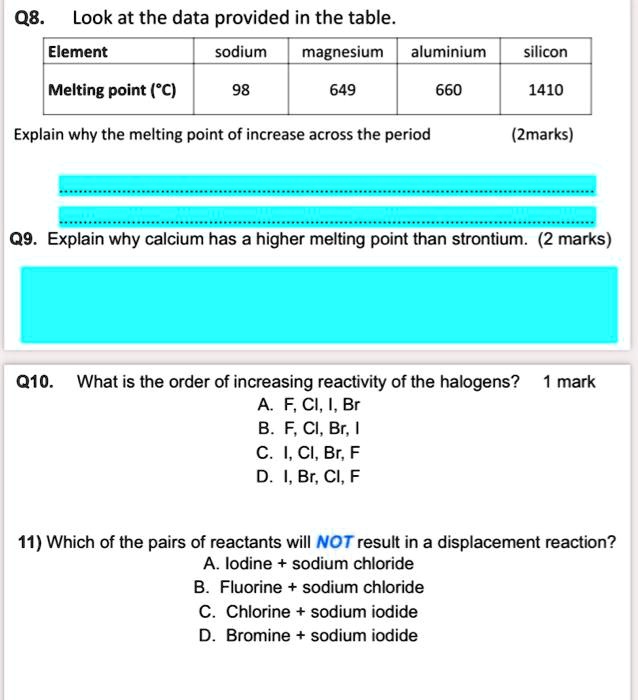
SOLVED: Look at the data provided in the table: Element sodium, magnesium, aluminium, silicon Melting point (°C): 98, 649, 660, 1410 Explain why the melting point increases across the period. (Z marks)



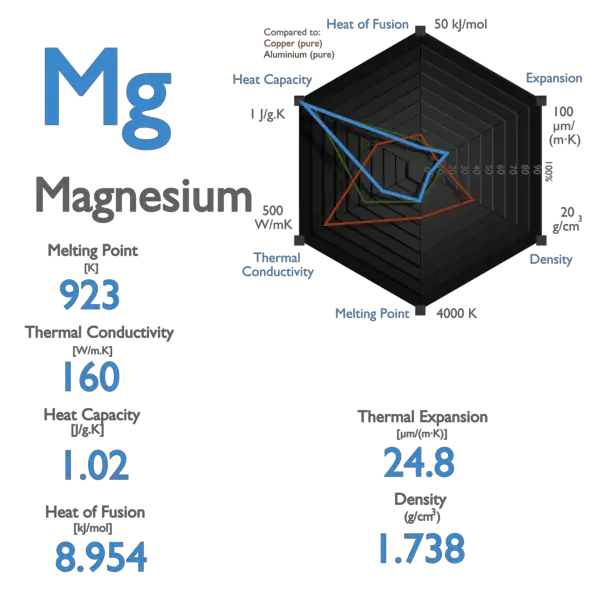



![Properties of pure and alloyed magnesium at its melting point [94]. | Download Table Properties of pure and alloyed magnesium at its melting point [94]. | Download Table](https://www.researchgate.net/profile/Vyasaraj-Manakari/publication/311957511/figure/tbl3/AS:614062450814976@1523415305341/Properties-of-pure-and-alloyed-magnesium-at-its-melting-point-94_Q320.jpg)
:max_bytes(150000):strip_icc()/GettyImages-1135707671-640473b29d534e15a24491c0d6b2789e.jpg)
![Properties of pure and alloyed magnesium at its melting point [94]. | Download Table Properties of pure and alloyed magnesium at its melting point [94]. | Download Table](https://www.researchgate.net/publication/311957511/figure/tbl3/AS:614062450814976@1523415305341/Properties-of-pure-and-alloyed-magnesium-at-its-melting-point-94.png)
