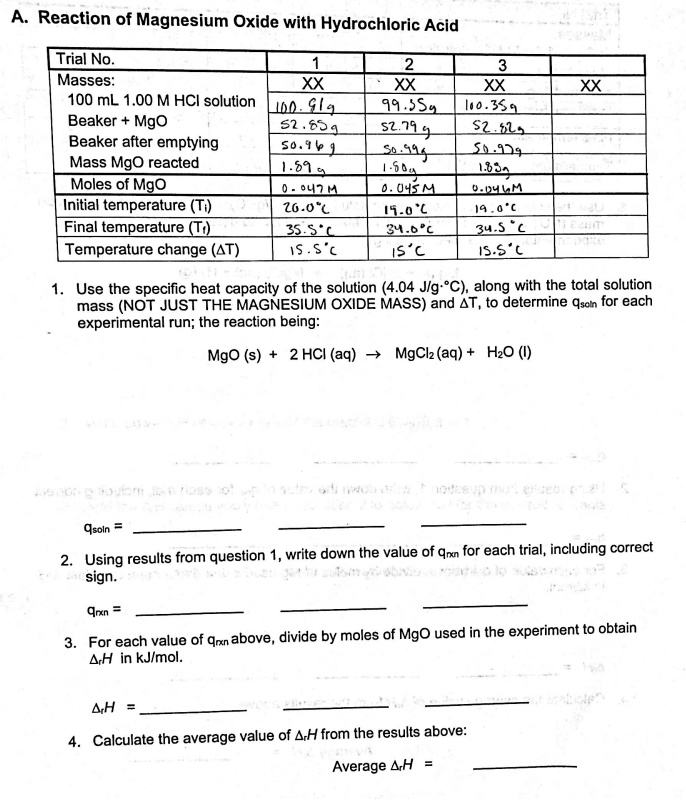
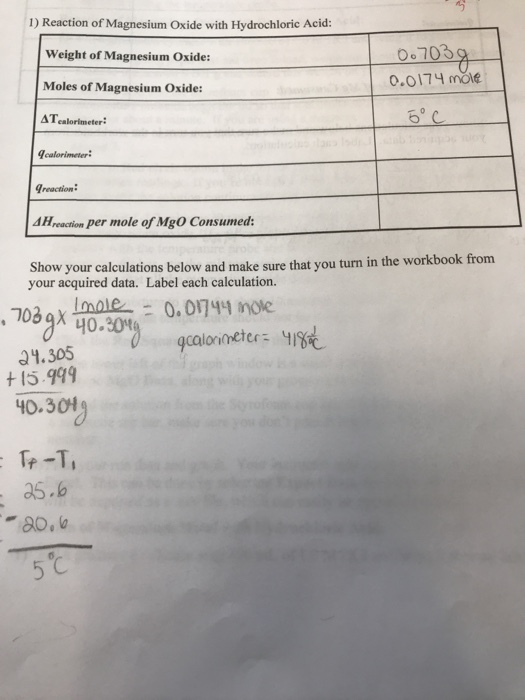
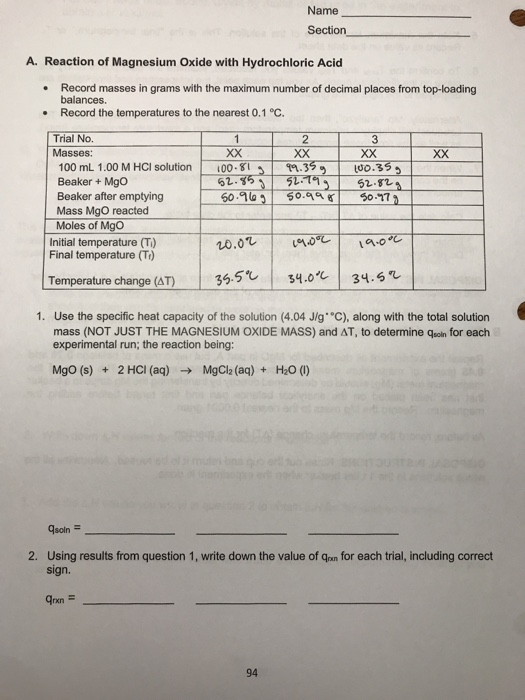
Malox, an over-the-counter antacid, contains aluminum hydroxide, Al(OH) 3, and magnesium hydroxides, Mg(OH). What are balanced equations for the reaction of both with stomach acid (HCl)? - Quora

Ionic equations A chemical equation shows the number of atoms and molecules of the reactants and products. Also shows physical state of reactants and products. - ppt download

16/10/2015 Acids and Bases Hydrochloric acidCitric acidWater An acid is a “proton donor”: A base is a “proton acceptor”: H Cl H +- H O Na H O - + Sodium. - ppt download

SOLVED: 2. Magnesium oxide, MgO, is not very soluble in water, and is difficult to titrate directly. Its purity can be determined by use of a 'back titration' method. 4.06 g of

Write the balanced chemical equations for the following reaction:Calcium hydroxide + Carbon dioxide → Calcium Carbonate + Water.

Introduction to Acids and Bases. Acid A substance that produces hydrogen ions, H + (aq), when it dissolves in water. Sour-tasting and good conductors. - ppt download
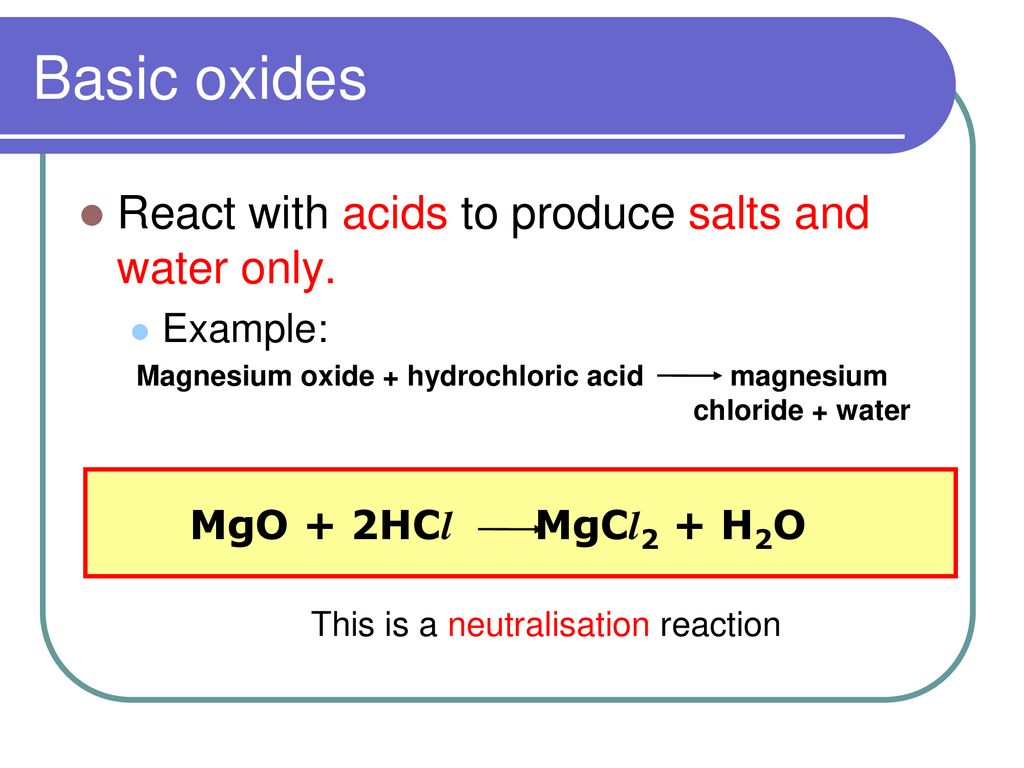
What is an oxide? An oxide is a Binary compound of oxygen and another element. M & O Oxides can be classified in two ways – Nature of Oxides Amount of. -
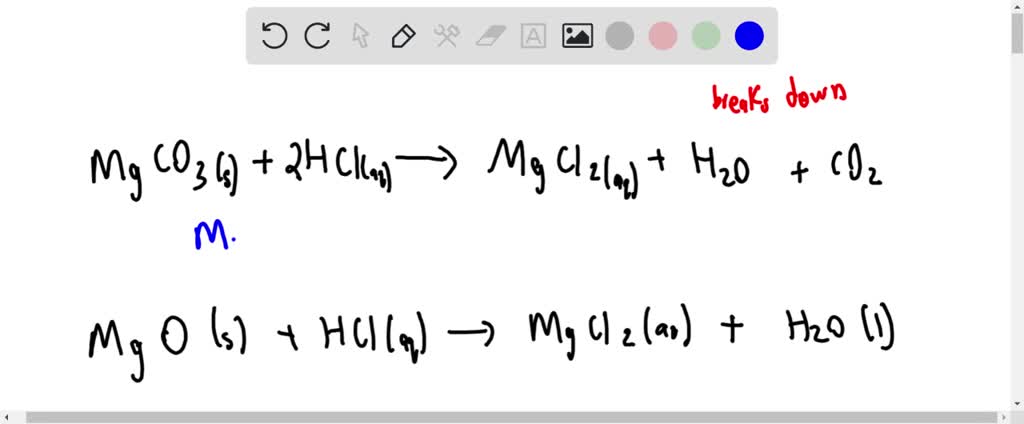
SOLVED:Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction that occurs