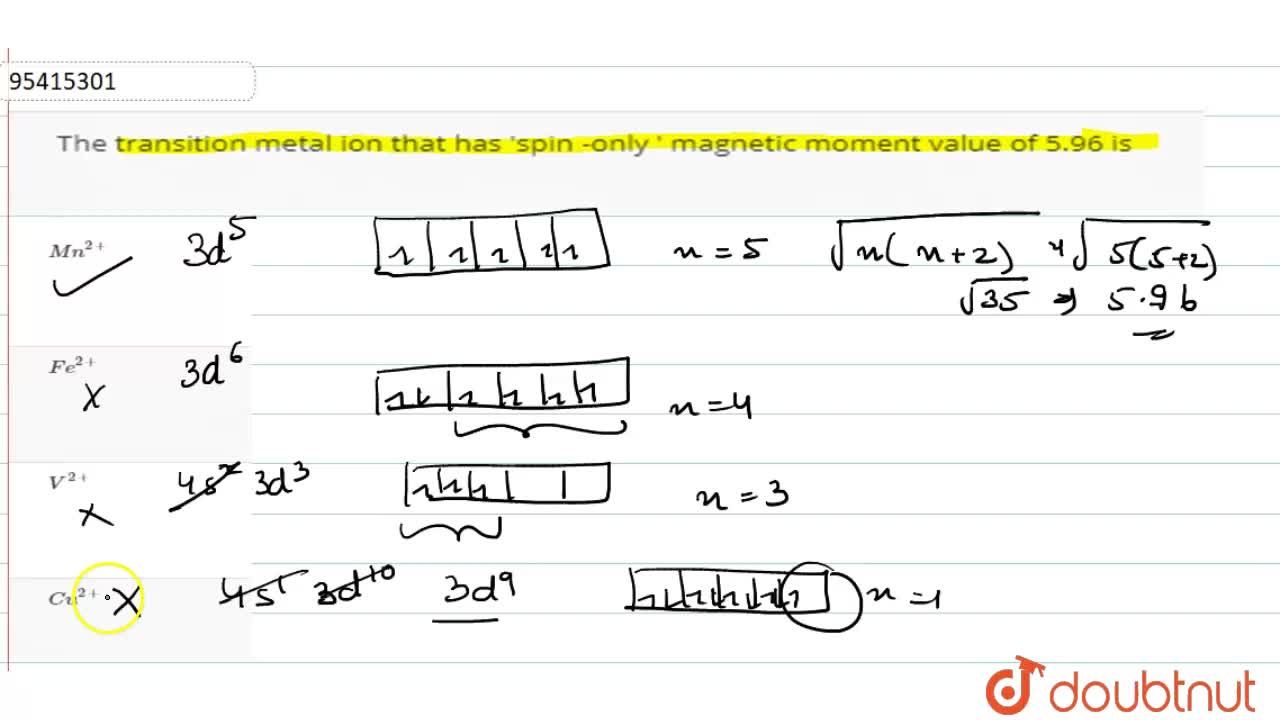
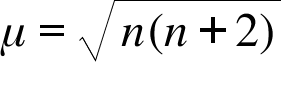What is the spin-only magnetic moment value (BM) of a divalent metal ion with atomic number 25, in - Sarthaks eConnect | Largest Online Education Community
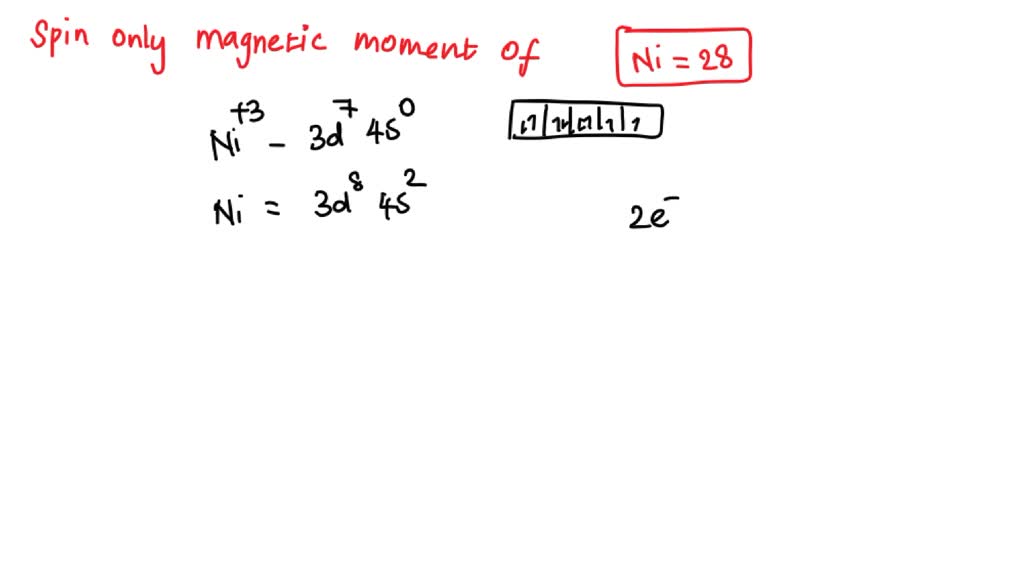
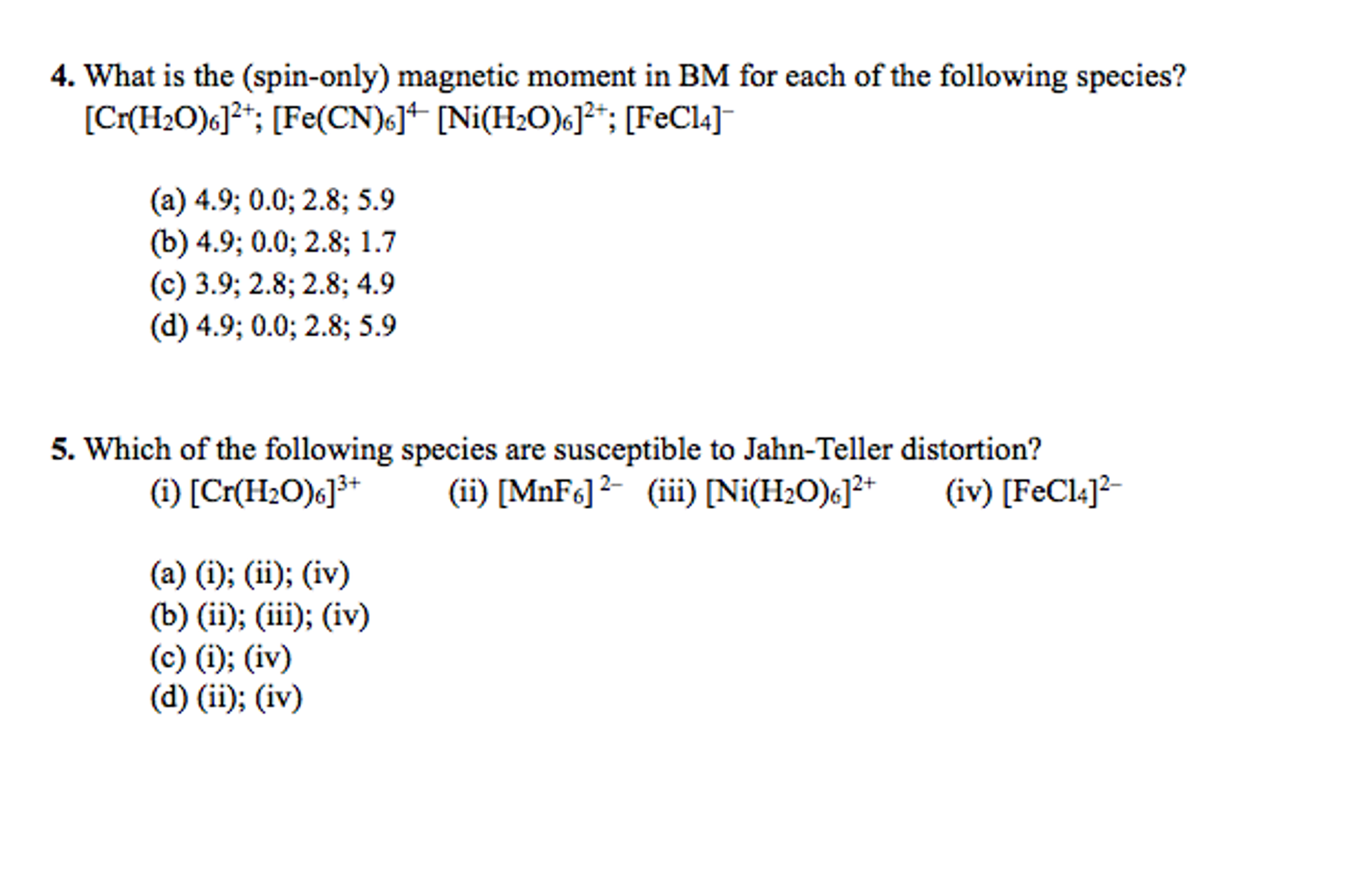
SOLVED: What is value of spin only magnetic moment of Ni (Z=28) in +3 oxidation state ? a)2.8 BM b) 3.1 BM c)0.0 BM d) 1.7 BM
Calculate the spin only magnetic moment of La^3+. - Sarthaks eConnect | Largest Online Education Community
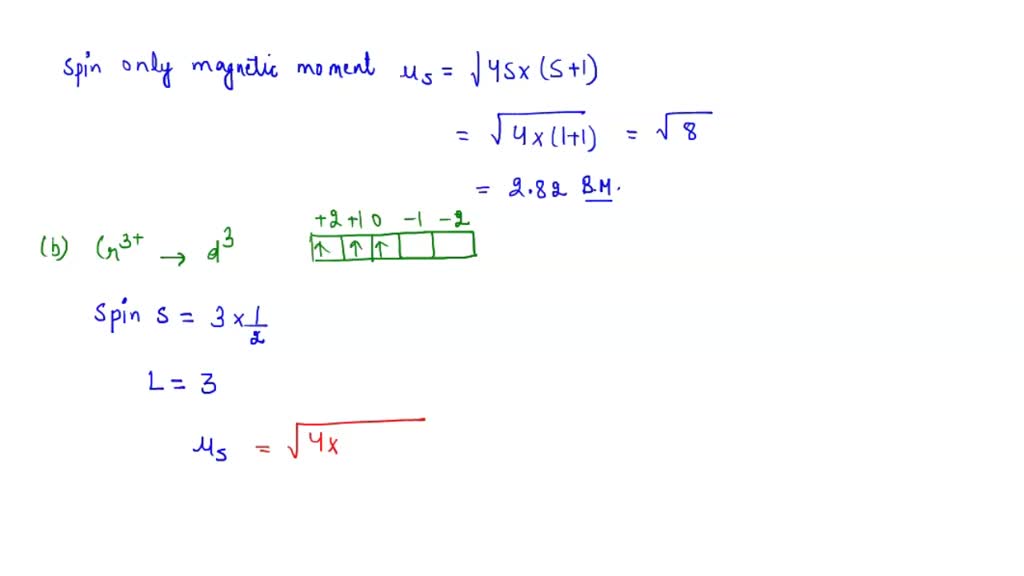
The value of the 'spin only' magnetic moment for one of the following configurations is 2.84 BM. The correct one is - Sarthaks eConnect | Largest Online Education Community

Write the spin only formula and give the unit of magnetic moment - Chemistry - Structure of Atom - 15663443 | Meritnation.com

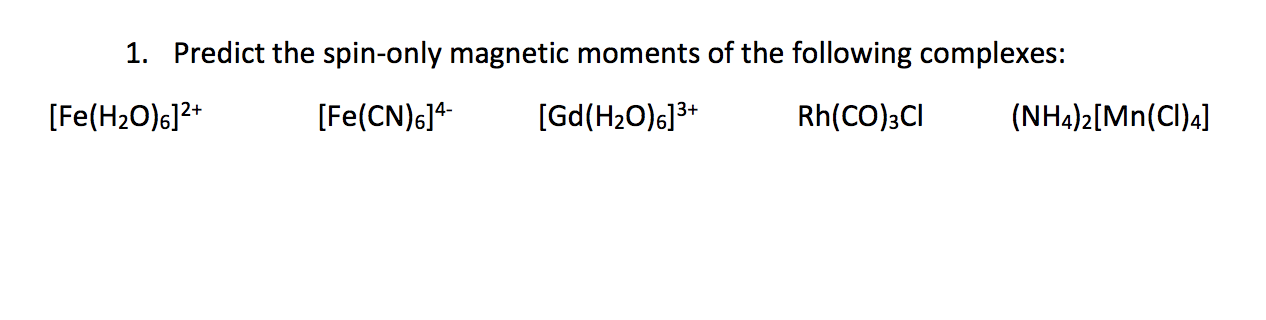
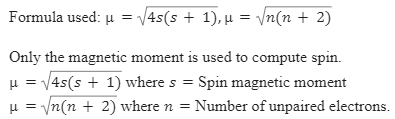





![The spin only magnetic moment of [CrF₆]⁴⁻ (atomic number for Cr is 24) is - NEETLab The spin only magnetic moment of [CrF₆]⁴⁻ (atomic number for Cr is 24) is - NEETLab](https://neetlab.com/wp-content/uploads/2017/11/The-spin-only-magnetic-moment-of-CrF-atomic-number-for-Chemistry-Question-.jpg)