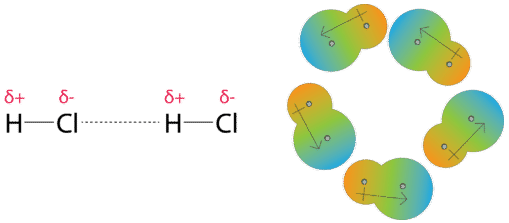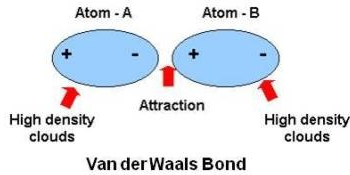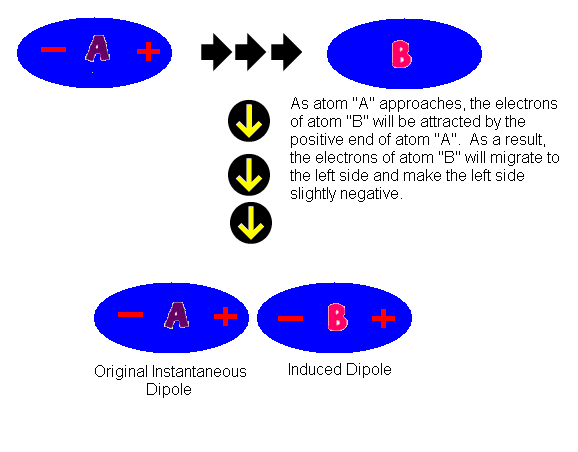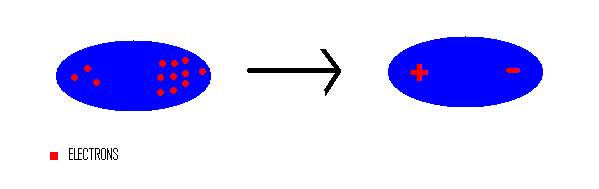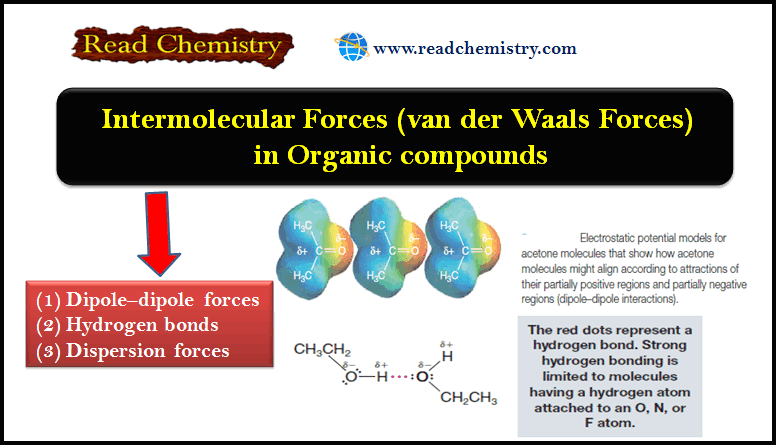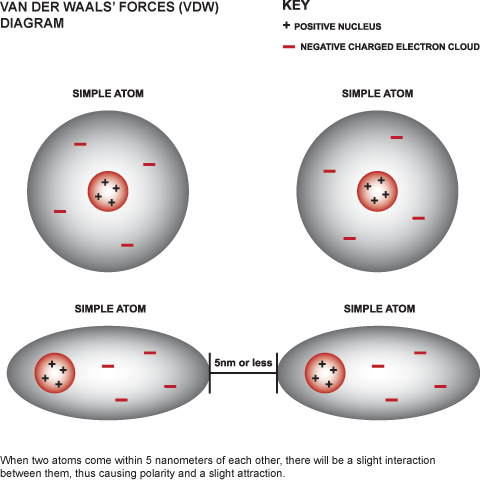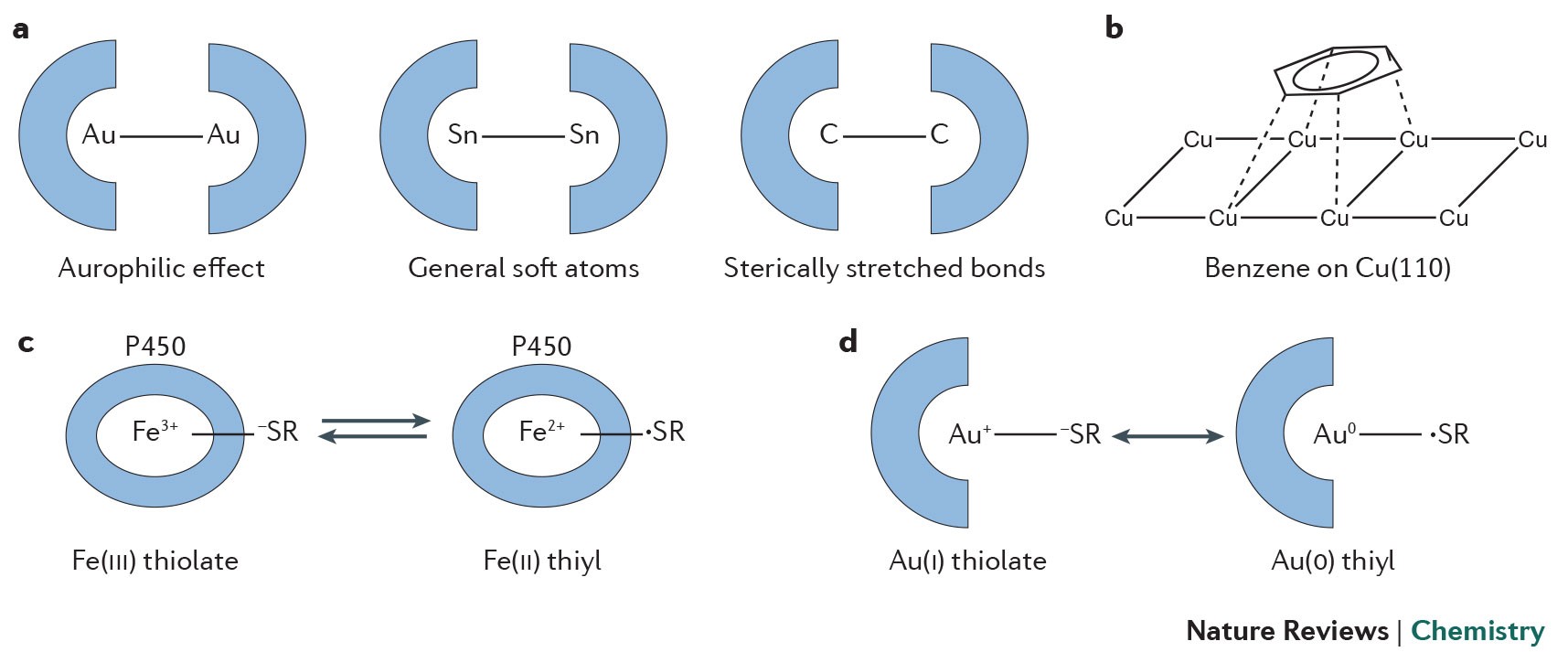
Competition of van der Waals and chemical forces on gold–sulfur surfaces and nanoparticles | Nature Reviews Chemistry

Van der Waals Emulsions: Emulsions Stabilized by Surface‐Inactive, Hydrophilic Particles via van der Waals Attraction - Marina - 2018 - Angewandte Chemie International Edition - Wiley Online Library

Open-science van der Waals interaction calculations enable mesoscale design and assembly | SDLE Research Center

physical chemistry - Which definition of van der Waals forces is correct? - Chemistry Stack Exchange

Difference Between Van der Waals and Hydrophobic Interactions | Compare the Difference Between Similar Terms

Van der Waals interactions and the limits of isolated atom models at interfaces | Nature Communications